Authors: Ivan Popov, Zhenghao Zhu, Harmandeep Singh, Catalin Gainaru, Stephen J. Paddison, and Alexei P. Sokolov
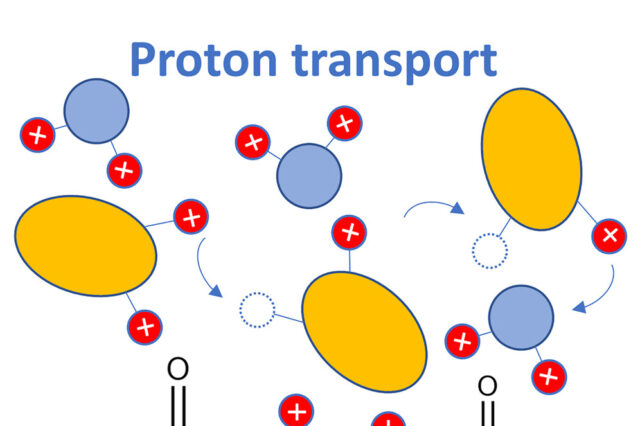
Abstract: The fundamental understanding of proton transport, specifically the Grotthuss-like mechanism, is critical for many technological applications. The present study presents a comprehensive analysis of proton transport in aqueous solutions of sulfuric and phosphoric acids using quasielastic neutron and light scattering, broadband dielectric spectroscopy, rheology, pulsed-field gradient-NMR measurements, and ab initio molecular dynamic simulations. Results show that in all systems, proton transport occurs through short “jumps” of ∼0.5 Å that are faster than structural relaxation. However, proton hopping appears to be coupled to structural relaxation in aqueous solutions of sulfuric acid, while these processes are decoupled in phosphoric acid. Neutron scattering indicates that all protons have the same fast mobility in phosphoric acid systems, while at least one proton per sulfuric molecule remains slower than other protons. Analysis reveals correlated proton jumps, but these correlations suppress conductivity, suggesting that expected Grotthuss-like enhancement of conductivity is unlikely in bulk liquids.